ANTIBIOTIC RESISTANCE
People shared two pictures of themselves side-by-side that were taken 10 years apart. In the posts they showed their accomplishments, how life had changed for them and even how they had changed as they became older.
But along with our lives, another organism's life changed too...
Over the last 10 years, bacteria have become resistant to our current antibiotics.

Barth, A. (2016, September 19). Rise of the Supergerms. Scholastic Science World.

Briers, Y. [@BriersYves]. (2019, January 19). #10YearsChallenge #antibiotic #Resistance [Tweet; image]. Twitter.

Barth, A. (2016, September 19). Rise of the Supergerms. Scholastic Science World.
#10YearChallenge?

What does it mean?
How did it happen?
What can we do?
What is antibiotic resistance?
A bacterial infection is like a group of invading soldiers attacking the body and making you sick. The antibiotics fight these bacteria. Depending on the class of antibiotics, different war tactics are used to target different aspects of the bacterial soldiers and disable them. When the antibiotics win, you recover. But these bacteria can become resistant to the tactics of antibiotics.
Watch me!
Antimicrobial resistance (AMR) is a worldwide crisis and on World Health Organization's list of 13 most urgent global health challenges in the next 10 years . Without antibiotics, minor bacterial infections and illnesses can become very dangerous, especially for people with already weaker immune systems like cancer patients. Surgeries, small or big, and dental procedures will carry a high risk of potentially deadly infection.

According to the World Health Organization, it is important:
-
For doctors to only prescribe antibiotics when necessary for bacterial infections.
-
To use antibiotics exactly as the prescription instructs.
-
Never demand antibiotics from a physician if not required.
-
Never share antibiotics with others or use old ones.
-
Fund research into the discovery of new classes of antibiotics and new tactics to fight bacteria.
A REAL-LIFE STORY...
Addie Rerecich
HOVER OVER ME!
5 months. That’s all the time it took for 11-year old Addie to have her life turn upside-down.
The Infectious Diseases Society of America (IDSA) recounts the story of Addie - a strong and athletic girl who loved to play softball and swim.
But with increasing pain in her hip and a fever that reached 103 degrees, Addie ended up in the emergency room and the next time she left the hospital she was in a wheelchair and had lost the ability to use her left arm and eye.
How did everything change so fast?
The discovery of antibiotics led to the Golden Age of the 20th century. They were the miracle drugs of the medicine world! Capable of treating infectious diseases, they soon became the foundation that built the American pharmaceutical industry. In fact, the discovery of antibiotics like penicillin in 1929 and streptomycin in 1943 coupled with improvements in hygiene practices, speculated the end of infectious diseases caused by bacterial infections.
But this did not happen.
As new antibiotics were produced, they were widely distributed and extensively used in humans and animals to treat diseases and even in livestock, fisheries and crops to promote growth. A recent observational study in 76 countries found that between 2000-2015 the consumption of antibiotics increased by 65%.
 |  |  |
|---|---|---|
 |
This overuse of antibiotics accelerated the development of resistant bacteria all too soon. According to Davies (2006), bacteria become resistant to new antibiotics within two-three years of their introduction.
With the start of the 21st century, antibiotic resistance has become a serious threat to global public health. The widespread use of antibiotics from hospitals to agriculture to animal farms, has led to a situation where bacteria are constantly trying to survive in different antibiotic-environments.
This has led to the development of new antibiotic-resistant bacterial strains called “superbugs” that are present all around us - from our playgrounds to our hospitals and even within our fruits, vegetables and meat.
Learn more about Addie's Story...
HOVER OVER ME!
An active child, Addie most likely got her initial bacterial infection, community-associated MRSA, from her community playground. Methicillin-resistant Staphylococcus aureus (MRSA) is a very difficult bacteria to treat as it is resistant to many commonly-used antibiotics.
Children easily get infected with this bacteria when they scrape their knees and arms while playing and then start picking on the scabs that form. “In Addie’s case, she was a skin picker. She, as do many kids, picked at her little scabs. And that was likely what introduced the [MRSA] infection” says Dr. Sean Elliot, an infectious disease specialist in Addie’s intensive care unit who was interviewed by Frontline - PBS for their documentary “Hunting the Nightmare Bacteria” released in 2013.
The Golden Age of antibiotic research peaked in the 1950s-1960s, during which new classes of antibiotics were continuously researched and introduced into the pharmaceutical market.
But, by the end of 1960s most research started focusing on altering existing natural antibiotics into stronger synthetic lab-made antibiotic compounds. This was beneficial in fighting bacterial infections more effectively and reduced the toxicity of these antibiotic compounds on the human body.
Yet, the steady decline in research focusing on discovering new antibiotic classes and the lack of innovation has led to a “discovery void”. Since the 1980s-1990s there was a reported 90% decrease in the discovery and treatment approval of new antibiotic classes.
The Center for Disease Dynamics, Economics and Policy have an antibiotic resistance map showcasing the resistant rates of different bacteria to different antibiotics.
Check the map out below:
Why did Addie's health worsen?
HOVER OVER ME!
Addie’s bacterial infection spread to her lungs. She got diagnosed with early pneumonia and antibiotic treatment was started. Unfortunately, a blood clot developed and broke off, blocking her right lung.
As her lungs worsened, her doctors placed her on an ECMO machine that would do the work of her lungs. Learn more about ECMO here.
But there was a hidden danger to this. The machine’s long tubes can harbor multiple antibiotic-resistant bacteria. “Any patient we put on ECMO has a much higher risk of having additional infections,” says Dr. Sean Elliot, “That’s just the nature of the beast.”
Addie’s weak lungs quickly acquired another bacterial infection - Stenotrophomonas maltophilia - that is naturally resistant to most antibiotics.
Unfortunately, this was not the end. During the course of her hospital stay, Addie acquired more antibiotic-resistant bacterial infections and most antibiotics could no longer treat those superbugs.
She was now in the post-antibiotic era.
Did You Know?
The extracorporeal membrane oxygenation (ECMO) machine is attached to a vein and an artery using long tubes that are connected to a pump. The machine takes blood from the vein, adds oxygen and removes carbon dioxide from it and sends the blood back to the heart via the artery.
A new Approach
Think of antibiotic resistance as a war between 2 armies: one being the antibiotics and the other being the bacteria.
It is a long-fought war. The antibiotic army mostly fights off the bacterial army.
One day, a bacterial soldier figures out the strategy of antibiotics.
This bacteria soldier passes on this information to all the new soldiers that join the bacteria army. Now bacteria start winning the war.
The antibiotic army will not win again unless they can develop a new strategy!
To win this war, it is crucial that we carefully plan our next attack strategy to defeat the bacterial army.
WHAT CAN OUR NEXT MOVE BE?
Researchers at McMaster University’s Wright Lab have developed a unique approach to developing new drugs to combat antibiotic resistance. Addressing the current global issue of antibiotic-resistant bacteria requires us to discover new strains of antibiotics with new mechanisms to combat the existing drug-resistant bacteria.
It was found throughout evolutionary history that biosynthetic gene clusters (BGC’s) were formed due to selective biological pressure, causing the resulting biosynthetic genes to have novel biological activity. Biosynthetic gene clusters are a grouping of all the physical genes that control responses for ecological interactions within plants.
Historically, new members in an antibiotic class using the same mechanism of actions were identified when using this method. Instead, identifying distinct BGC's, it would be possible to find new antibiotics with different methods.
A lot of antibiotics available in the pharmaceutical market are produced from bacteria of the actinomycetes family. Antibacterial medication is made by screening the actinomycetes. It was found that screening this bacterium is unfavorable, as it only causes the rediscovery of known antibiotic compounds. Genome sequencing of the actinomycetes reveals a large quantity of BGC’s, but we need to initialize prioritization to find with of these newly discovered clusters holds new chemical matter. With this chemical matter, predictions are made to assess which members of the glycopeptide family of antibiotics would contain new methods to combat bacteria. This method allowed for the discovery of 2 species of a functional class of glycopeptide. The first one being an already known glycopeptide antibiotic “complestatin” and a new compound, named “corbomycin”.
Current antibiotics function by blocking the formation of the bacterial cell wall. While the newly discovered complestatin and corbomycin are the first known compounds that prevent the breakdown of the bacterial wall. The breakdown of the bacterial wall is necessary for cell multiplication, but due to the wall being unable to be broken down, the bacteria cannot reproduce.

Bacteria have a wall around the outside of their cells that gives them shape and is a source of strength. The antibiotics that we found actually work by doing the opposite – they prevent the wall from being broken down. If you completely block the breakdown of the wall, it is like it is trapped in a prison, and can’t expand or grow.
– Bethany Culp, PhD candidate in Biochemistry and Biomedical Sciences at McMaster University.
Finding compounds that can perform this method of antibiotic action was long sought over by microbiologists. Corbomycin and complestatin were found to be effective against multidrug-resistant bacteria and they show low potential for bacterial resistance development.
These two new compounds are an exciting discovery for future antibacterial development.
This new approach could be the 'new strategy' the antibiotic army needs to start winning against the bacteria!
BUT What does this mean for us?
Since these compounds kill the bacteria in a way that has never been seen before, antibiotic medications created using these compounds can “kill bacteria that are resistant to other antibiotics” - Elizabeth Culp, a PhD candidate in Biochemistry and Biomedical Sciences at McMaster University.
Seems like our antibiotic-resistant issue is solved, right?
There is still a lot more research that needs to be done before these compounds can be useful to humans. They still need to figure out "how it will travel through the bloodstream, its reaction in the body, and if it will be able to reach the target site when ingested orally" - Elizabeth Culp, a PhD candidate in Biochemistry and Biomedical Sciences at McMaster University
WRONG!
Listen to FULL INTERVIEW here:
A different Approach?
To have better understanding, Freddie converses with a doctor about possible treatments and solutions to antibiotic resistance. Elizabeth Culp, a researcher at McMaster University, mentions that researchers keep finding similar antibiotics as a result the research in antibiotics is not progressing. Instead, researchers should focus on phage therapy which is one of the only possible treatments that are effective and can be used to replace antibiotics.
Freddie
So, doctor what are some of the other possible solutions to avoid antibiotic resistance?
Freddie, to avoid the crisis of antibiotic resistance, there is a new option of replacing antibiotics with bacteriophages which are a stronger group of soldiers that destroy the enemy in a much safer way. This is called "Phage therapy" and it has been used as an antibacterial agent since 1996.
Freddie
Why is this therapy good?

Some of the benefits of phage therapy over antibiotics are that bacteriophages target specific enemies so it does not cause harm to other cells in the body. Also, once they reach the war site, they multiply into a larger army which makes them stronger and easier for them to fight their enemies. Lastly, the process of making these bacteriophages is natural and it does not harm the environment.

Freddie
How do doctors use phage therapy in hospitals?
Well, Freddie, the phage therapy technique has been used in hospitals in France since 1919 to treat all sorts of diseases. But they stopped this process in 1979 because researchers and doctors found that antibiotics were easier, effective and accessible for everyone. But as I have mentioned before, the use of antibiotics for a longer period of time can lead to bacteria becoming resistant to it and eventually the antibiotics will not have any effect on the enemy.
Instead, doctors can use vaccines to inject phages into the bloodstream which takes 2-4 hours and 10 hour to reach the target organ. Also, the effect of phage stays in the body for several days.
But phage therapy has not been approved in Canada. But countries in Europe like Georgia and Poland use phage therapy. Right now, there are no new inventions of drugs that can effectively treat the disease and day by day they are becoming useless. Even though bacteriophages are safe to use, we still need to do more research on this.
Overall, bacteriophages will not only help in the medical field but will also help with antibiotic resistance in the agricultural field. So, there are more advantages of using bacteriophages than disadvantages especially compared to antibiotics.

Freddie
Thank you for answering all the questions! I wonder how the future is going to be...
100 years in the future if no new antibiotics are discovered
If no new antibiotics are discovered, all of the bacteria on earth will slowly but steadily become resistant to the current antibiotics. These antibiotics will become ineffective and fail to treat bacterial infections. If there is no medication that can be used to treat these bacterial infections, we will "go back to an era when infectious disease was the main killer" - Elizabeth Culp, a PhD candidate in Biochemistry and Biomedical Sciences at McMaster University.
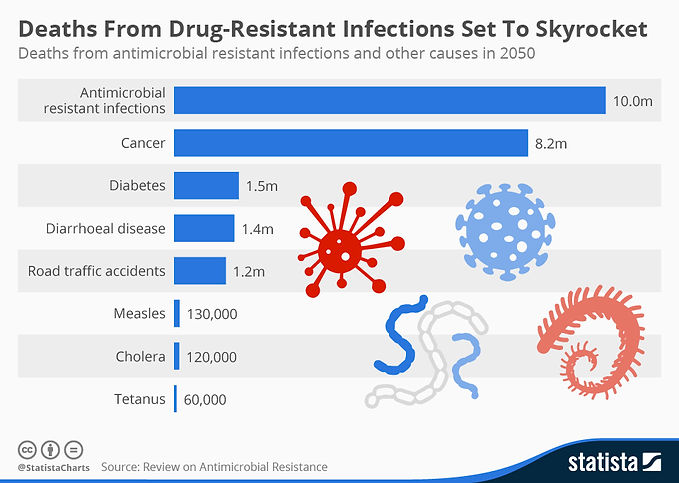
In 2019, more than 35,000 people in the United States died from antibiotic-resistant infections. One study estimated that almost 300 million people will die over the next 35 years (starting from 2014) because of antibiotic-resistant infections.
This is what the numbers will look like worldwide

Click on these topics to learn more about antibiotic resistance and current initiatives:
References
Note: Pictures and icons that are not explicitly cited in the references section are from the Wix stock of images.
Background Picture: News and Events. (2016, May 24). Gut bacteria may predict chemotherapy risk. https://twin-cities.umn.edu/news-events/gut-bacteria-may-predict-chemotherapy-risk
Title Picture: Biology lab GIF by University of California. https://giphy.com/gifs/uofcalifornia-growth-bacteria-3osxYeymtCD76SeQjC
Introduction:
Alanis, A. J. (2005). Resistance to antibiotics: are we in the post-antibiotic era? Archives of
medical research, 36(6), 697-705.
Barth, A. (2016, September 19). Rise of the Supergerms. Scholastic Science World. https://scienceworld.scholastic.com/issues/2016-17/091916/rise-of-the-supergerms.html
Briers, Y. [@BriersYves]. (2019, January 19). #10YearsChallenge #antibiotic #Resistance [Tweet; image]. Twitter. https://twitter.com/briersyves/status/1086734750344187907
CDC. (2018, November). Worried your sore throat may be strep? https://www.cdc.gov/groupastrep/diseases-public/strep-throat.html
CDC. (2020, June 18). Biggest Threats and Data. https://www.cdc.gov/DrugResistance/Biggest-Threats.html
Fernando, G. A., Collignon, P. J., & Bell, J. M. (2010). A risk for returned travellers: the
“post-antibiotic era”. Medical Journal of Australia, 193(1), 59.
WHO. (2020, January 13). Urgent health challenges for the next decade. https://www.who.int/news-room/photo-story/photo-story-detail/urgent-health-challenges-for-the-next-decade?utm_source=STAT+Newsletters
WHO. (2020, July). Antibiotic resistance. https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance
A Real-Life Story (Addie's Story):
bioMérieux Connection. (2018, July 12). How to Explain Antimicrobial Resistance to Your Friends and Family (With Infographics). https://www.biomerieuxconnection.com/2018/07/12/explain-antimicrobial-resistance-friends-family-infographics/
Davies, J. (2006). Where have All the Antibiotics Gone? The Canadian Journal of Infectious Diseases & Medical Microbiology, 17(5), 287–290. doi: 10.1155/2006/707296
Young, R. (Producer). (2013). Hunting the Nightmare Bacteria [Film]. Frontline - PBS.
Klein, E. Y., Boeckel, T. P. V., Martinez, E. M., Pant, S., Gandra, S., Levin, S. A., Goossens, H., & Laxminarayan, R. (2018). Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. PNAS, 115(15), 3463–3470. https://doi.org/10.1073/pnas.1717295115
Lawler, P.R., Silver, D.A., Scirica, B.M., Couper, G.S., Weinhouse, G.L., & Camp, P.C. (2015). Extracorporeal Membrane Oxygenation in Adults With Cardiogenic Shock. Circulation, 131(7), 676-680. https://doi.org/10.1161/CIRCULATIONAHA.114.006647
Luepke, K.H., Suda, K.J., Boucher, H., Russo, R.L., Bonney, M.W., Hunt, T.D., & Mohr III, J.F. (2016). Past, Present, and Future of Antibacterial Economics: Increasing Bacterial Resistance, Limited Antibiotic Pipeline, and Societal Implications. Pharmacotherapy, 37(1), 71-84. https://doi.org/10.1002/phar.1868
Lynm, C. (n.d.). [Cartoon of bacteria that undergo evolution from constant antibiotic exposure] [Photograph]. Zox. http://anarchak.com/article/40290/jmn130117fa
Meredith. (2017, July 26). Antibiotics: excessive use in livestock and why it is a problem. Feed Them Wisely. https://feedthemwisely.com/antibiotics-in-livestock
Parker, D. (2020, August 10). New insights on antibiotics use on crops amongst smallholder farmers. BioMed Central. https://blogs.biomedcentral.com/bmcblog/2020/08/10/new-insights-on-antibiotics-use-on-crops-amongst-smallholder-farmers/
ReAct Group. (n.d.). Few antibiotics under development. https://www.reactgroup.org/toolbox/understand/how-did-we-end-up-here/few-antibiotics-under-development/
Schmidt, F. (n.d.). Herd health: agriculture’s role in the global AMR crisis. Pharmaceutical Technology. https://www.pharmaceutical-technology.com/features/animal-antibiotics/attachment/animal-antibiotic-stats-credit-flo-schmidt/
The Center for Disease Dynamics, Economics and Policy. (2020). Resistance Map. https://resistancemap.cddep.org/AntibioticResistance.php
Williams, A. (2018, November 16). Should the meat industry cut out antibiotics? Food Navigator. https://www.foodnavigator.com/Article/2018/11/16/Animal-antibiotics-use-in-the-spotlight
A New Approach:
Biotecnika. (2020, February 13). Unique Antibiotic Against Antibiotic Resistant Bacteria Developed. https://www.biotecnika.org/2020/02/new-antibiotic-for-antibiotic-resistant-bacteria-corbomycin/
Culp, E. J., Waglechner, N., Wang, W., Fiebig-Comyn, A. A., Hsu, Y. P., Koteva, K., ... & Wright, G. D. (2020). Evolution-guided discovery of antibiotics that inhibit peptidoglycan remodelling. Nature, 578(7796), 582-587. https://doi-org.libaccess.lib.mcmaster.ca/10.1038/s41586-020-1990-9
OpenStax College Biology. (n.d.). Prokaryotic Cells: Figure 1. RICE University. https://cnx.org/contents/GFy_h8cu@9.87:pOpVdIwp@11/Prokaryotic-Cells
Poitras, C. (2015, February 5). Getting Ahead of Antibiotic-resistant Bacteria. UConn Today. https://today.uconn.edu/2015/02/getting-ahead-of-antibiotic-resistant-bacteria/
A Different Approach:
Doctor Icon: Icon made by Freepik from www.flaticon.com
Golkar, Z., Bagasra, O., & Pace, D. G. (2014). Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. The Journal of Infection in Developing Countries, 8(02), 129-136.
Future:
Statistic Graph: https://www.statista.com/chart/3095/drug-resistant-infections/
O'Neill, J. (2014). Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations [Ebook]. Review on Antimicrobial Resistance. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf.
Worldwide deaths attributed to AMR every year by 2050 image taken from: Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations
Pictures not from Wix: